eCRF / ePRO
practice-proven. modular.
innovative.
eCRF / ePRO
practice-proven.
modular.
innovative.
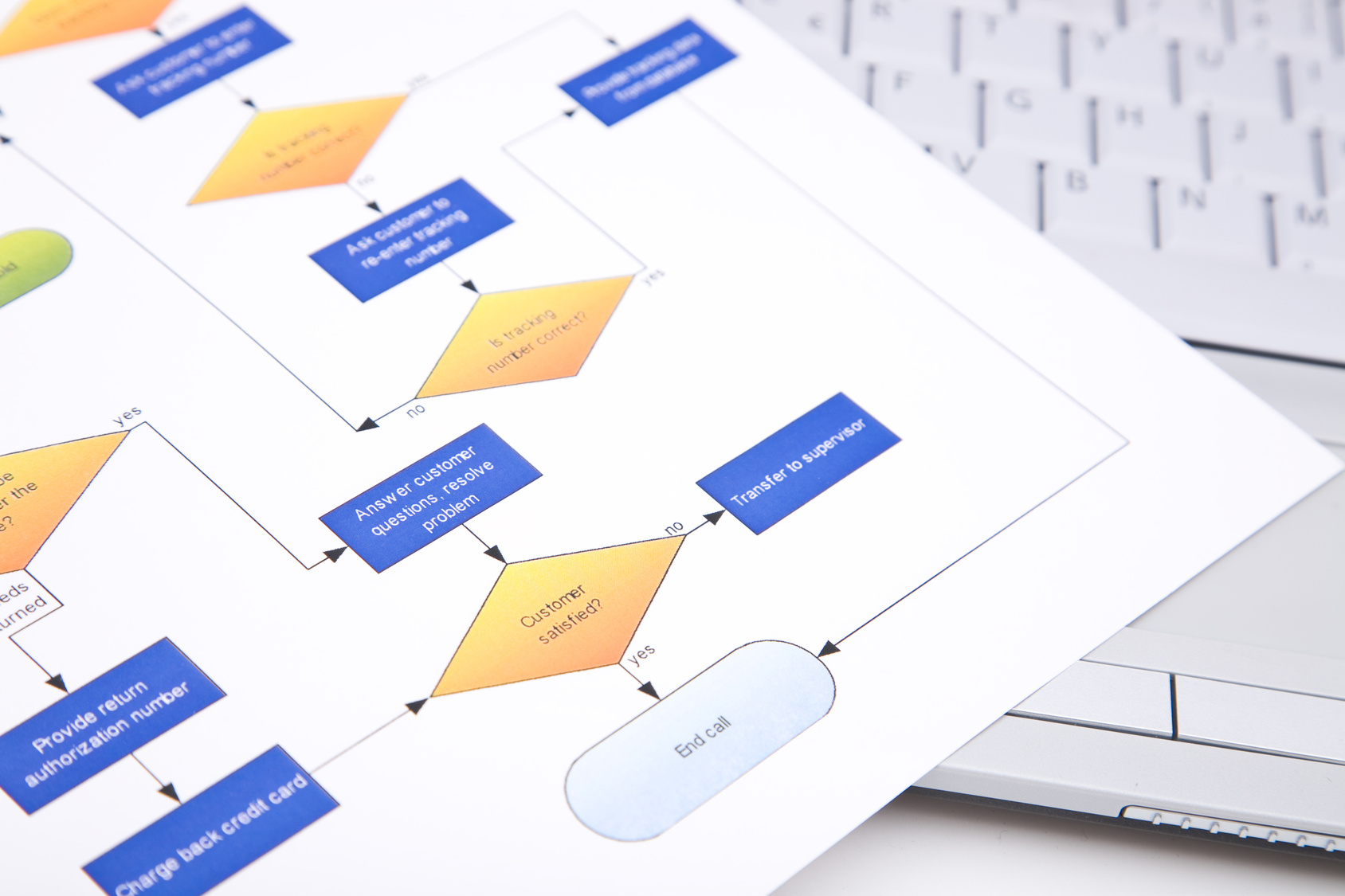
The MedSurv-Portal

Who we are
We provide our customers an validated online EDC system (eCRF/ ePRO) for data capture in:

How we work
We accompany our customers from the planning of the eCRF to the validation of the study-specific requirements and ensure a structured project workflow.
On request, we can offer a full service with reliable and internationally active partners, such as CROs, data managers, statisticians, biometrists, etc.
What makes us
We offer our costumers
Our quality results from our certified management system. We are constantly developing ourselves and our user-friendly tools.

Who we are
We provide our customers an validated online EDC system (eCRF/ ePRO) for data capture in:

How we work
We accompany our customers from the planning of the eCRF to the validation of the study-specific requirements and ensure a structured project workflow.
On request, we can offer a full service with reliable and internationally active partners, such as CROs, data managers, statisticians, biometrists, etc.

What makes us
We offer our costumers
Our quality results from our certified management system. We are constantly developing ourselves and our user-friendly tools.
Compliance
CDISC
US FDA
21 CFR
Part 11
EU GxP
Annex 11
FDA Industry
Guidelines
GAMP 5
ICH-GCP
Guidelines
CDISC
US FDA
21 CFR
Part 11
EU GxP
Annex 11
FDA Industry
Guidelines
ICH-GCP
Guidelines
GAMP 5
Modules
Audit Trail
Tracking of changes according to legal requirements
Randomisation
Customisable algorithm
Reliable ratio
(S)AE Management
(Serious) adverse events are automatically processed
Monitoring
Virtual data check
Current patient and visit status
Management of the centres
Data Management
Additional validation process according to DVP
Query Management
Direct communication in the portal
ePRO/ eDiary
Direct collection of questionnaires (QoL)
Patient diary
Document upload
Uploading of documents with patient allocation
Clinical Trial Management
Daily updates of study data
Transparency
Remuneration
Recruitment status…
News

Authority inspection passed with "good"
On November 24th and 25th, 2021, we had two inspectors from Regional Council of Darmstadt for inspection according to § 64 AMG with focus on § 16 GCP-V in our house.
The first sentence in the final discussion was analogously “MedSurv and the MedSurv-Portal are and will continue to be suitable for carrying out or accompanying clinical trials”.

Our new eQuestionnaire
Product Questionnaire
Do you know the new medical device regulation?
Do you want or have to carry out PMCF studies?
Just contact us at +49 6187 905 91 47 or sales@medsurv.de.
For more informations: MS eQuestionnaire, Survey e

We train as a partner
of the IHK Hanau!
After the positive experiences of the past few years, we are once again offering three young people the opportunity to train as IT specialists.
In addition to the theoretical training in school, they are onvolved in practical work from the very beginning.

Our new eDiary/ ePRO
Patient Questionnaire
Are paper diaries still up to date?
Test our solution!
For more information, please click on the following link: MS ePRO, eDiary e

Authority inspection passed with "good"
On November 24th and 25th, 2021, we had two inspectors from Regional Council of Darmstadt for inspection according to § 64 AMG with focus on § 16 GCP-V in our house.
The first sentence in the final discussion was analogously “MedSurv and the MedSurv-Portal are and will continue to be suitable for carrying out or accompanying clinical trials”.

Our new eQuestionnaire
Product Questionnaire
Do you know the new medical device regulation?
Do you want or have to carry out PMCF studies?
Just contact us at +49 6187 905 91 47 or sales@medsurv.de.
For more informations: MS eQuestionnaire, Survey e

We train as a partner
of the IHK Hanau!
After the positive experiences of the past few years, we are once again offering three young people the opportunity to train as IT specialists.
In addition to the theoretical training in school, they are onvolved in practical work from the very beginning.

Our new eDiary/ ePRO
Patient Questionnaire
Are paper diaries still up to date?
Test our solution!
For more information, please click on the following link: MS ePRO, eDiary e
Testimonials
Testimonials
“The database appears very clear and self-explanatory. I am already looking forward to entering the database.”
Verena Zähringer, Study Coordinator and head of the non-medical study area Angiology II, University Medical Center Freiburg - University Heart Center
„We would like to take this opportunity to thank you and your team again for the wonderful cooperation over the past years.
With your company, we have a great and reliable team at our side. Thanks a lot for this!”
Vanessa Pingel, Borstel Medical Clinic, Study Center, Leibniz Lung Center
“MedSurv offers a reasonable price-performance ratio. Study-specific requirements are implemented in very high quality.
In addition, MedSurv convinces by promptly answering all questions regarding the eCRF.
Last but not least, we highly appreciate the constant striving for improvement.”
SocraMetrics GmbH Team, Erfurt
“The MedSurv portal is user-friendly, secure access limited and can be adapted very flexible to individual needs for supporting even a complex study design. Our multi-year and ongoing collaboration (in phase III studies) is characterized by open communication and competent project management.”
Beate Schmitz, Head of Clinical Trial Management, Biofrontera Bioscience GmbH, Leverkusen
“Since 2000, I have conducted several scientific studies on the epidemiology of infections together with MedSurv GmbH via the MedSurv portal. Collaboration with Mattes Hamann’s team has always worked brilliantly. I particularly appreciate the stability of the web applications, the high security standard in the log-in area for the authorised members (e.g. doctors), as well as the uncomplicated and very timeley implementation of any changes and updates.”
Prof. Dr. med. Arne Simon, University Clinic of Saarland, Clinic for paediatric Oncology and Haematology, Homburg
“We have had very good experience with MedSurv GmbH. The drawing up of a validated database for our Phase II/III study proceeded rapidly; corrections were made quickly and as desired (even during the ongoing study). Overall, collaboration with MedSurv is very uncomplicated and consistently enjoyable. User support in the sites was also uncomplicated and fast. We can only recommend the MedSurv team.”
Ruxobeat Study Team, Uniklinik RWTH Aachen, Aachen
“The planning and implementation of the eCRF for our study according to MPG §23b was very professional and cooperative.”
Dr. med. Christian Herzmann, Senior Physician, Research Center Borstel - Leibniz Lung Center, Clinical Study Center
“The NIS is uncomplicated, well-thoughtful and very well implemented eletronically. Coordination with MedSurv runs perfectly.”
Dr med. Tobias von Kuegelgen, Investigator, Urologie Wedel, Wedel
“We value MedSurv as a reliable and competent partner. Their good accessibility, friendly communication and their dedicated support in particular are always praised by our study staff.“
Dr. Simone Nüdling, Managing Director, med:unit GmbH, Cologne
“The user-friendly interface of the MedSurv portal is very helpful in our multicentre study.
Changes and updates are possible at any time during the current project and are implemented promptly. User support works without any inconvenience across the borders of Germany.”
The iPegasus study team, University Medical Center Hamburg-Eppendorf, Hamburg
“When it comes to project management, the MedSurv team gets full points.”
Julia Pache, Manager Medical Operations, Biogen GmbH, Munich
“In our study, MedSurv GmbH was always an approachable, reliable partner, who responded flexibly to our wishes.”
Dr. Claudia Neumeister, Senior clinical research project manager, Dr. Pfleger Arzneimittel GmbH, Bamberg
“The database appears very clear and self-explanatory. I am already looking forward to entering the database.”
Verena Zähringer, Study Coordinator and head of the non-medical study area Angiology II, University Medical Center Freiburg - University Heart Center
„We would like to take this opportunity to thank you and your team again for the wonderful cooperation over the past years.
With your company, we have a great and reliable team at our side. Thanks a lot for this!”
Vanessa Pingel, Borstel Medical Clinic, Study Center, Leibniz Lung Center
“MedSurv offers a reasonable price-performance ratio. Study-specific requirements are implemented in very high quality. In addition, MedSurv convinces by promptly answering all questions regarding the eCRF. Last but not least, we highly appreciate the constant striving for improvement.”
SocraMetrics GmbH Team, Erfurt
“The MedSurv portal is user-friendly, secure access limited and can be adapted very flexible to individual needs for supporting even a complex study design. Our multi-year and ongoing collaboration (in phase III studies) is characterized by open communication and competent project management.”
Beate Schmitz, Head of Clinical Trial Management, Biofrontera Bioscience GmbH, Leverkusen
“Since 2000, I have conducted several scientific studies on the epidemiology of infections together with MedSurv GmbH via the MedSurv portal. Collaboration with Mattes Hamann’s team has always worked brilliantly. I particularly appreciate the stability of the web applications, the high security standard in the log-in area for the authorised members (e.g. doctors), as well as the uncomplicated and very timeley implementation of any changes and updates.”
Prof. Dr. med. Arne Simon, University Clinic of Saarland, Clinic for paediatric Oncology and Haematology, Homburg
“We have had very good experience with MedSurv GmbH. The drawing up of a validated database for our Phase II/III study proceeded rapidly; corrections were made quickly and as desired (even during the ongoing study). Overall, collaboration with MedSurv is very uncomplicated and consistently enjoyable. User support in the sites was also uncomplicated and fast. We can only recommend the MedSurv team.”
Ruxobeat Study Team, Uniklinik RWTH Aachen, Aachen
“The planning and implementation of the eCRF for our study according to MPG §23b was very professional and cooperative.”
Dr. med. Christian Herzmann, Senior Physician, Research Center Borstel - Leibniz Lung Center, Clinical Study Center
“The NIS is uncomplicated, well-thoughtful and very well implemented eletronically. Coordination with MedSurv runs perfectly.”
Dr med. Tobias von Kuegelgen, Investigator, Urologie Wedel, Wedel
“We value MedSurv as a reliable and competent partner. Their good accessibility, friendly communication and their dedicated support in particular are always praised by our study staff.“
Dr. Simone Nüdling, Managing Director, med:unit GmbH, Cologne
“The user-friendly interface of the MedSurv portal is very helpful in our multicentre study. Changes and updates are possible at any time during the current project and are implemented promptly. User support also works without any inconvenience across the borders of Germany.”
The iPegasus study team, University Medical Center Hamburg-Eppendorf, Hamburg
“When it comes to project management, the MedSurv team gets full points.”
Julia Pache, Manager Medical Operations, Biogen GmbH, Munich
“In our study, MedSurv GmbH was always an approachable, reliable partner, who responded flexibly to our wishes.”